Mini Superstars for Aquatic Research: Stable Isotopes
Published March 29, 2022

Monitoring the nation’s water quality is a fundamental part of EPA’s work. As part of the Clean Water Act, EPA monitors the condition of U.S. waters through a program called the National Aquatic Resource Surveys (NARS). This is a collaborative program between EPA, states, and Tribes designed to assess the quality of the nation's coastal waters, lakes, reservoirs, rivers, streams and wetlands.
To conduct part of this monitoring, EPA scientists get help from some very small assistants – stable isotopes. Isotopes are atoms of the same element that have the same number of electrons and protons, but different numbers of neutrons. The difference of one teeny tiny neutron can reveal big differences when it comes to evaluating the nation’s water. Stable isotopes measured within NARS samples (water, insects, soils) help researchers determine many things about water quality and the surrounding environment. Stable isotope ratios integrate, indicate, record, and trace fundamental processes in hydrology and ecology. Stored within the atoms of NARS samples, stable isotope ratios of specific elements unlock stories about ecological processes that impact water quality and quantity.
The Equipment
EPA’s Integrated Stable Isotope Research Facility (ISIRF) in Corvallis, Oregon, uses four isotope-ratio mass spectrometers to measure the isotopic ratios of ecologically important elements such as carbon (C), nitrogen (N), hydrogen (H), and oxygen (O). These instruments can measure the difference of one atomic weight even when one isotope is much more abundant than the other. The heavier isotope moves more slowly and bonds more strongly than the light isotope, so differences in the isotopic ratio of the two depict measurable differences in how the isotopes cycle in the environment.
Renée Brooks, a researcher at the Corvallis lab, describes it like this: the heavier isotope tends to stay behind when the elements are cycling in the environment, so heavier isotopic ratios generally reflect some reaction that has occurred. The trick is figuring out which reaction has taken place. Isotopes don’t lie, but we need to figure out what they are saying.
Applications
EPA Researchers have used a variety of isotopes to understand changes in aquatic ecosystems and water quality. A good example of teasing out the isotopic story inside the atoms comes from the nitrogen atoms inside aquatic insects and how they help tell the tale of nutrient pollution. The nitrogen isotope ratio (15N/14N, denoted as δ15N) in insects can relay information about the source of nitrogen, and about ecosystem processes that remove it. Fertilizer is a common input of nitrogen into the environment and has a distinct δ15N value. In large amounts, nitrogen in fertilizer becomes a major threat to aquatic ecosystems. Removal processes increase the δ15N value and reduce nitrogen moving to aquatic ecosystems. Measuring the δ15N in aquatic insects along with the total nitrogen concentration in streams helps identify places where fertilizer is moving into streams unimpeded, and places where ecosystems are cycling and removing much of that nitrogen before it reaches the aquatic systems.
Nitrogen isotopes can also be measured in wetland soils to identify wetland hotspots of nitrogen processing. This processing links wetland condition to important ecosystem services such as denitrification, a key process for removing nitrogen from water. Soil isotopes provide an efficient, low-cost means to measure nitrogen removal by wetlands on large scales.
Isotope ratios of water (H and O isotopes) can show how characteristics of lakes and reservoirs change over time and across landscapes. One of these characteristics is evaporation and aids in understanding lake drawdown. Measurements of the difference in lake levels and water isotopes can inform management strategies to identify water bodies that are particularly vulnerable to changing water use demands and increasing drought conditions.
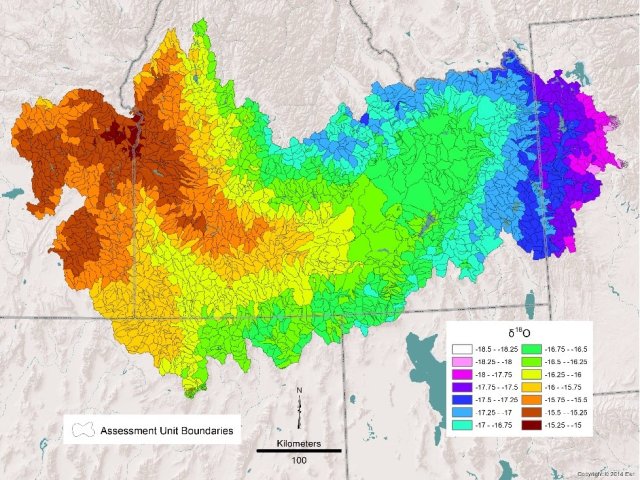
Using isotopes of oxygen and hydrogen from water, “isoscapes” can be painted of major river basins. Since isotopes of precipitation vary with latitude, longitude and elevation, isoscapes show where water originates within the basin (See figure 1). As snowpacks at high elevation decrease with climate change, it’s important to know the sources of water contributing to rivers. High elevation cold-water inputs to rivers are important for endangered salmon runs. It’s also important for agriculture and reservoir management to determine availability of snowpack melt to keep the rivers flowing for spring and summer irrigation.
Stable isotopes are a mighty atomic tool that has applications across several research areas. Measuring the stable isotopic ratio of biologically important elements from samples collected in NARS provides indicators of key ecological processes that influence those elements and the associated aquatic environments. It is a low-cost and efficient means to measure critical stressors in environments that affect human health.
For the Future
What’s next for this impressive research tool? EPA is looking to expand this analysis with these current research projects:
- Determining nutrient pollution in seagrass
- Studying how wildfires affect major rivers
- Understanding harmful algal bloom dynamics on lakes
- Using carbon isotopes to measure tree physiological responses to climate change and extreme weather events
- Discovering how water use, land-development, and climate change affect stream flows
Interested in learning more about stable isotopes and how they might help your research? Feel free to reach out to the ISIRF team.
Renee Brooks: brooks.renee@epa.gov
Lisandra Trine: trine.liasndra@epa.gov
Learn more:
Meet EPA Ecohydrologist J. Renée Brooks, Ph.D.
Already into isotopes? Learn more about measuring isotopes.
