Toxicokinetics Overview
Toxicokinetics: How Does the Body Process a Chemical?
Chemical disposition studies are performed to determine what happens to a substance in an organism after that organism is exposed. Absorption, distribution, metabolism, and excretion (referred to as ADME) studies evaluate the fate of a substance in an organism following exposure. Here are some ways to think about ADME:
- Absorption: How does the chemical get absorbed into the body tissues?
- Distribution: Where does the chemical go inside the body?
- Metabolism: How do enzymes in the body break apart the molecules of a chemical?
- Excretion: How does the chemical leave the body?
Integrating toxicokinetic data into toxicity studies can improve the quality of chemical risk assessment in many ways, including aiding in the understanding of differences in responses or sensitivity between individual animals, species, or life stages, and supporting the extrapolation of findings in experimental animals to humans. The use of toxicokinetics can also help to reduce the use of vertebrate animals used in chemical testing, which supports the Agency's New Approach Methods Work Plan.
On this page:
- What is Toxicokinetics?
- Toxicokinetic Models
- Physiologically-Based Toxicokinetic (PBTK) Models
- Toxicokinetics Data and Tools
- Related Information
What is Toxicokinetics?
Toxicokinetics (TK) is the study describing ADME in a biological system following exposure. TK links external exposure concentration to the internal dose and determines what occurs to the substance within the system.
-
Internal dose: the concentration of a substance in one or more systems of interest.
TK describes the rates of ADME (i.e., the rate at which a substance enters a biological system and what occurs to the substance once it is in the system), allowing researchers to estimate internal dose from the external exposure concentration, or using reverse toxicokinetics, to calculate the estimated external exposure concentration from the known internal dose.
Toxicokinetic Models
Toxicokinetic models are representations of "compartments" that chemicals flow through. TK models describe ADME mathematically by representing the body through these compartments and flows. The amount of chemical in each compartment (vs. time) is described using a mass balance equation. TK models can be simple, empirical models (featuring only one or two compartments), or more detailed physiologically based models (PBTK). It is important to note that TK models do not predict toxicity, but they can help researchers understand how much of a substance might be present in an organ where toxicity is predicted or observed through experiments or other models. PBTK models are commonly used in EPA's Rapid Chemical Exposure and Dosimetry Research.
Physiologically-Based Toxicokinetic Models
Physiologically-Based Toxicokinetic (PBTK) models feature multiple compartments and flows that represent real physiological quantities.
Note: PBTK models may also be referred to as Physiologically Based Pharmacokinetic (PBPK) models, as pharmacokinetics (PK) is a synonym for toxicokinetics (TK). They are often used interchangeably. PK can be used to connote pharmaceuticals while TK can be used to connote environmental chemicals.
While PBTK models have more parameters overall (such as an organ's volume, volume of blood, or lung capacity), most of them do not need to be estimated from concentration vs. time data. With PBTK models, parameters representing physiological quantities are known a priori based on existing anatomy studies. For example, we would know that the volume of the liver compartment would equal the actual known volume of the liver.
Estimations in PBTK Models
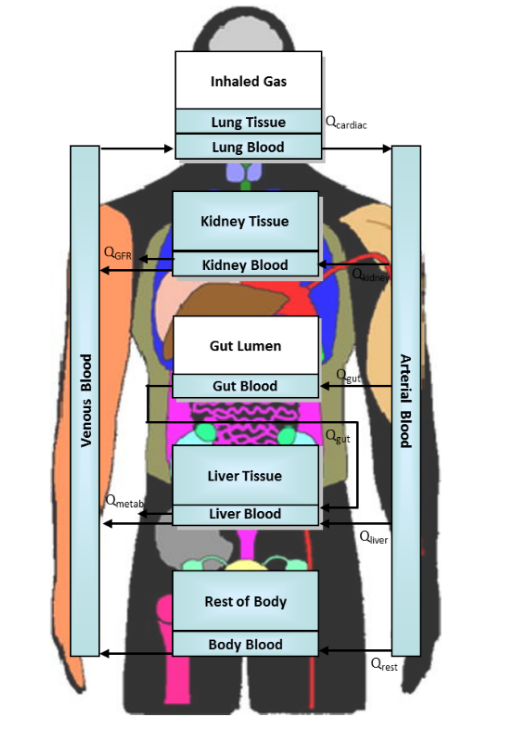
When using PBPK models to study a new substance of interest, some parameters need to be estimated. These include parameters representing chemical-body interactions. For example, we might need to estimate the rate of hepatic metabolism of chemical (i.e., How fast does the liver break down the chemical?).
How are PBTK Models Helpful?
PBTK models may be either specifically developed to describe detailed ADME processes for a given chemical, or they may be used for generic application, meaning it is the same for all chemicals.
PBTK models are also particularly useful for extrapolation compared to TK models, such as:
- Inter-species extrapolation (e.g., animals to humans)
- Learn more about reducing the use of vertebrate animal testing: New Approach Methods (NAMs) Work Plan
- Route-to-route extrapolation (e.g., dermal exposure to oral exposure)
- Internal-external extrapolation (i.e., reverse toxicokinetics - internal dose to external exposure concentration)
- Extrapolation to similar chemicals (e.g., similar chemical structure)
- By keeping the same generic model for chemicals and substituting in different chemical-specific parameters (such as structural or functional qualities), researchers gain the ability to make TK predictions quickly for a large number of similar chemicals. Learn more about TK chemical extrapolation: High-Throughput Toxicokinetics (HTTK).
- In vitro-in vivo extrapolation (IVIVE) (i.e., conducting studies outside of the body to extrapolate results for inside the body)
- In vitro studies are performed with microorganisms, cells, or biological molecules outside their normal biological context, such as using cultures of lung tissue when testing inhalation exposure. In vivo refers to studies in the body, like using animal research to study inhalation exposure. IVIVE can reduce the need for animal testing by extrapolating results from in vitro studies.
Toxicokinetics Data and Tools
high-throughput toxicokinetics (httk) R Package
EPA researchers developed toxicokinetic models within an R software package called high-throughput toxicokinetics (httk) to estimate chemical concentrations in humans. The package currently uses human in vitro data to make predictions about the fate of chemicals in humans, rats, mice, dogs, and rabbits.
To make useful predictions about chemical risks facing the public, scientists need three types of data: exposure, dosimetry, and hazard. Exposure is the amount of a chemical one is exposed to, dosimetry is the way the chemical interacts with one’s body, and hazard is the amount of a chemical that causes toxicity.
Access: httk: High-Throughput Toxicokinetics R Package
EPA scientists plan to continue improving the accuracy of the HTTK R Package. New models are being developed to describe absorption through the skin, exposure to aerosols (clouds of droplets), partial oral absorption, and human pregnancy.
