Electronic Submissions of Pesticide Applications
Status of Front End Processing
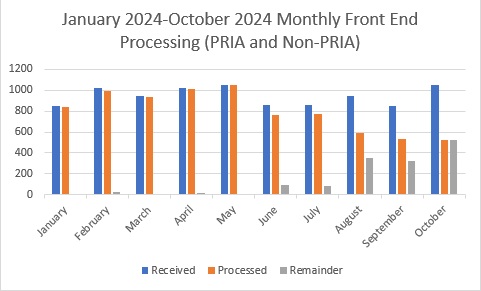
From January 1st, 2024 through October 30th, 2024, approximately 9,435 packages (PRIA and Non-PRIA) have been received, approximately 8,010 packages were processed, and approximately 1420 remain to be processed with these being roughly split about 1% PRIA and 99% Non-PRIA. Approximately 20% of the Non-PRIAs are represented by notifications or fast track amendments. The figure below represents these numbers by month.
- Please contact Rachel Holloman and Raderrio Wilkins (holloman.rachel@epa.gov and wilkins.raderrio@epa.gov) if you have questions regarding a specific front-end processing question.
General Information about Electronic Submission of Pesticide Applications
Applications for pesticide registration can be submitted electronically, including forms, studies, and draft product labeling. Applicants need not submit multiple electronic copies of any pieces of their applications.
- In PR Notice 2011-3, EPA made clear that the requirement to submit multiple copies of data is applicable only to paper submissions.
- Similarly, EPA interprets the requirement to submit five copies of draft labeling in 40 CFR 152.50(e) to apply only to applications made on paper.
As electronic submissions are easily reproducible, EPA will accept electronic applications containing one copy of all the required elements.
The Pesticide Submission Portal (PSP) is accessed through EPA’s Central Data Exchange (CDX) Network and requires user registration. Registrants currently submitting CDs or DVDs using the e-Dossier downloadable tool or their own builder tools based on EPA’s XML guidance may use the portal and forego the courier costs of sending to EPA.
Updates to the Electronic Submission Portal
EPA has expanded the portal to allow voluntary data submissions related to specific registration review cases. As elsewhere in the PSP, voluntary data submissions will support real-time validations, status updates, and email notifications to ensure a streamlined experience. Users can submit the following data voluntarily via the new "Voluntary Submission" link on the PSP home page:
- Study citations.
- Form 8570-35 data matrices.
- Submission cover letters.
- Studies (protocols, study profiles, supplemental study data).
The most recent release of the portal, PSP 1.4, allows users to resubmit previously submitted 90-day responses. Once a 90-day response or data submission has been successfully transmitted to OPP, users may perform the following actions:
- Change responses to data requirements.
- Cite additional studies.
- Upload additional documents.
- Change how the product registration is supported.
In addition to the above changes, PSP 1.4 introduces a number of enhancements and bug fixes, including:
- An "OPP Status" column on the "DCI List" screen that describes the status of the DCI in OPP's system.
- A list of required documents/data is located next to each "Registrant Response" option within DCIs. Users will now know at a glance what information is required for a particular response.
- A fix for Admin Numbers and Source EPA Registration Numbers erroneously appearing in the copy of record.
- A number of technical fixes and performance improvements related to large submission packages.
Electronic Submission Categories
We encourage electronic submissions for the following regulatory actions:
- New pesticide active ingredients
- New pesticide products containing already-registered pesticide active ingredients
- Amendments to registered pesticide products
- Experimental use permits
- Inert ingredient requests
- Pre-application
- 6(a)(2) data
- Petitions for food tolerance
- Distributor products
- Adding an alternate brand name
- Cancelling a distributor product
- Cancelling all distributor products
- Reinstating a distributor product
- Data Call-Ins (DCIs)
- Acknowledgment of receipt of DCI (new DCIs issued after release of Phase 2 only)
- Initial 90-day response to DCI (new DCIs issued after release of Phase 2 only)
- Data submissions responding to DCI requirements (for all DCIs)
- Resubmit previously submitted 90-day responses. Once a 90-day response or data submission has been successfully transmitted to OPP, users may perform the following actions:
- Change responses to data requirements.
- Cite additional studies.
- Upload additional documents.
- Change how the product registration is supported.
- Voluntary submissions
- Study citations.
- Form 8570-35 data matrices.
- Submission cover letters.
- Studies (protocols, study profiles, supplemental study data).
EPA will continue to accept DCI-response submissions via paper but encourages applicants to take advantage of this new, more efficient option.
- Supplemental information submitted at Agency request.
- Submissions of data as a condition of registration.
Application Materials
- Forms for pesticide registration applications.
- Supporting data may be required as part of the submission.
- Draft labeling that meets the regulatory requirements set out in 40 CFR 152.50.
- Also see the Label Review Manual, in particular, Chapter III. General Labeling Requirements.
- Regulations for labeling requirements (40 CFR 156)
Tools for Developing an Electronic Submission
- The New Pesticide Submission Portal (PSP) within EPA's Central Data Exchange (CDX) Network.
- Electronic Submission Assistance: User guides, e-Dossier, and XML guidance.
- Guidance on study formatting and supplemental files and review aids.
- Electronic submission of labels.
- Resources for test order recipients (Endocrine Disruptor Screening Program information on “How to Submit Info in Response to Orders”, MRIDs, guidance and other resources).
Contacts for more information/assistance on e-submission issues.
